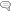The contact process is based on the equilibrium between Sulfur dioxide and its oxidation product sulfur trioxide:

which is only attained at a suitable rate in the presence of a catalyst. Since this equilibrium shifts in favor of the starting materials with increasing temperature, the process has to be carried out at the lowest temperature possible, this being determined by the optimal temperature of the catalyst. A higher sulfur dioxide conversion can be obtained by lowering the concentration of the sulfur trioxide formed (double contact process) or by operating under increased pressure (5 bar) (Ugine-Kuhlmann process).
During the Contact Process for manufacturing sulphuric acid, sulphur dioxide must be converted into sulphur trioxide. To convert sulphur dioxide into sulphur trioxide, solid vanadium(V) oxide catalyst is used.
The sulphur dioxide is oxidised to sulphur trioxide by the vanadium(V) oxide. Vanadium(V) oxide is reduced to vanadium(IV) oxide.
The vanadium(IV) oxide is then re-oxidised by the oxygen to form solid vanadium(V) oxide. The catalyst was changed slightly due to the property of transition metals.
The emission of sulfur dioxide from sulfuric acid plants is strongly reduced with the double contact process.The catalytic oxidation of sulfur dioxide to sulfur trioxide can also be carried out in a fluidized bed reactor. The gas to be converted is fed in at the bottom of a fluidized bed containing the catalyst in the form of abrasion resistant beads. The whole fluidized bed can be kept at a suitable temperature by the removal of the heat of reaction with a pipe cooler. This thermal mode of operation enables gases with a higher content of sulphur dioxide to be processed and more compact plants to be built.
In this process hydrogen sulfide, from coking plants, is converted to sulfur dioxide and water with an excess of air:
and the moist sulfur dioxide catalytically oxidized to sulfur trioxide. The high water content of the gas would result in only 75 to 78% sulfuric acid can be produced in this process. In coking plants this is generally reacted with the ammonia produced during coking gas purification to form ammonium sulfate. In newer processes the water vapor is largely condensed allowing sulfuric acids with a content of up to 98% to be produced.

http://www.lookchem.com/Chempedia/Chemical-Technology/Inorganic-Chemical-Technology/2976.html
http://www.chemguide.co.uk/physical/catalysis/introduction.html
http://boyisitsmokyinhere.blogspot.sg/2009/11/e1-air-pollution.html
http://boyisitsmokyinhere.blogspot.sg/2009/11/e1-air-pollution.html
Labels: ccsulphurdioxide

 Monday, 24 December 2012
Monday, 24 December 2012